The Osia® System, which launched commercially in 2020, is indicated for people with conductive hearing loss, mixed hearing loss and single-sided sensorineural deafness (SSD). The new Osia System with Osia OSI300 Implant enables patients to undergo MRI at 3.0 Tesla (T) without the need for surgery. This has been made possible by the OSI300’s unique Piezo Power™ technology and next-generation 3.0 T magnet technology
[Lone Tree, Colo.] – August 18, 2023: Cochlear Limited (ASX: COH), the global leader in implantable hearing solutions, introduces its next generation Cochlear™ Osia® System with the ability to have an MRI at 3.0 T, designed to improve hearing outcomes for people with conductive hearing loss, mixed hearing loss and single-sided sensorineural deafness (SSD).
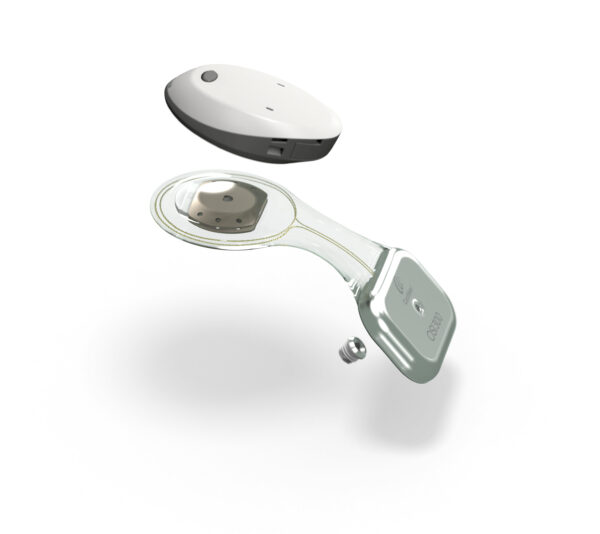
The new Osia System offers the proven benefits as the current version: excellent hearing outcomes1, ease of use, discreetness and reliability2. With the introduction of the OSI300 Implant, it is the first and only active bone conduction system that allows patients to undergo MRI scans at both 1.5 T and 3.0 T without the need for surgery3. This new patient benefit is made possible by combining the unique properties of the Osia System’s Piezo Power™ transducer, which does not contain magnetic material, and the next generation in implant magnet technology.
The OSI300 Implant is specifically designed to support access to MRI with no performance degradation after MRI exposure4,5, as there is nothing to be demagnetized as in an electromagnetic transducer. Unlike an axial magnet, which is found in most bone conduction implants today and is unsuitable for a patient undergoing an MRI examination at 3.0 T, the OSI300 uses a diametric magnet that sits within a casing and rotates to align with the magnetic field of the MRI machine.
“Not only does the new design allow for MRI scans at 3.0 T without surgery or the need for a headwrap or splint kit; we have also designed the magnet cassette to be simple to remove if needed. The easy removal of the implant magnet reduces image artifact, an important consideration for maximum visibility of areas around the implant. Both features mean less MRI preparation time and less time without sound, which is beneficial to both recipients and professionals,” Ryan Lopez, Head of Portfolio Strategy & Professional Marketing at Cochlear, said.
Today, most people can expect to undergo an MRI scan at some point in their lifetime and having a hearing implant shouldn’t be an obstacle to this important healthcare treatment option.
“The use of 3.0 T scanners is increasing and becoming more common. 3.0 T scanners help in the proper diagnosis of many neurologic, orthopedic and oncology conditions. It is important that patients don’t face any obstacles to this type of care, and I am glad Cochlear has kept this in mind in their new Osia System,” Dr. Brian Kaplan, SVP, Global Clinical Strategy & Innovation at Cochlear, Chairman of the Department of Otolaryngology, and Director of the Cochlear Implant Program at the Greater Baltimore Medical Center, said.
Cochlear’s product portfolio is inspired by meaningful innovation and a core belief that technology is only as useful as the benefit it provides.
“An improvement in the ability of patients to undergo high-resolution MRI scans with our technology was the number one request we heard from customers. With the new system, patients with an active bone conduction system can conveniently undergo MRI at 1.5 T and at 3.0 T with no impact to their hearing – MRI should be accessible to everyone” says Dig Howitt, Chief Executive Officer & President of Cochlear.
Launched commercially in 2020, the Osia System is the world’s first active osseointegrated bone conduction (OSI), a new category of bone conduction hearing solutions that uses digital piezoelectric stimulation to bypass damaged areas of the natural hearing system to send sound vibrations directly to the inner ear (cochlea).
Commercial release
The next generation of the Osia System will be available in clinics across the United States this fall and is currently being reviewed by Health Canada. Availability in other countries is subject to regulatory approvals.
For further information on the Osia System, visit cochlear.us/OsiaMRI-PR.
 About Cochlear Limited (ASX: COH)
About Cochlear Limited (ASX: COH)
Cochlear is the global leader in implantable hearing solutions. The company has a global workforce of more than 4,000 people and invests more than AUD$180 million each year in research and development. Products include cochlear implants, bone conduction implants and acoustic implants, which healthcare professionals use to treat a range of moderate to profound types of hearing loss.
Since 1981, Cochlear has provided more than 700,000 implantable devices, helping people of all ages, in more than 180 countries, to hear. www.cochlear.com
Christy LaPlante, PR Manager
E: pr-cochlearamericas@cochlear.com
References
-
-
- Mylanus EAM, Hua H, Wigren S, et al. Multicenter Clinical Investigation of a New Active Osseointegrated Steady-State Implant System. Otol Neurotol. 2020;41(9):1249-1257. OSI100 Implant and Osia Sound Processor;B P110 Power on Softband
- Osia Reliability Report Flyer, Cochlear Limited. 2023; D1841762.
- Ellsperman SE, Nairn EM, Stucken EZ. Review of Bone Conduction Hearing Devices. Audiol Res. 2021;11(2):207-219.
- OSI300 1.5 T MRI Verification Summary Report. Cochlear Limited, Australia. 2023; D2084831.
- OSI300 3 T MRI Verification Summary Report. Cochlear Limited, Australia. 2023; D2084832.
-
- In the US, the Osia system is cleared for children 12 years and older. In Canada, the Osia system is approved for children 5 years of age and older.